
1984年,厦门大学生物系,学士学位;
1991年,美国德克萨斯大学西南医学中心,生物化学博士;
1991年-1995年,美国Howard Hughes Institute at UCSD,博士后;
1995年-2001年,新加坡国立大学分子与细胞生物研究所,实验室主任;
2001年-2006年,香港科技大学生化系助理教授、副教授(获终身职位); 兼职于厦门大学生命科学学院,任长江学者特聘教授;
2003年12月-2017年5月,任厦门大学生命科学学院院长;
2006年6月至今,厦门大学生命科学学院,教授、博士生导师;
2021年,中国科学院院士。
1984: B.S. Xiamen University;
1991: Ph.D., UT Southwestern Medical Center at Dallas;
1991-1995: Postdoctoral Fellow at the Howard Hughes Medical Institute, UCSD;
1995-2001: Principal Investigator, IMCB, Singapore; 2001-2006: Assistant Professor/Associate Professor (tenured), Hong Kong University of Science and Technology;
2003.12-2017.5: Dean, School of Life Sciences, Xiamen University;
2006.6-Present: Professor, Cheung Kong Scholar, Xiamen University;
2021:member of CAS
研究领域(Research Area)
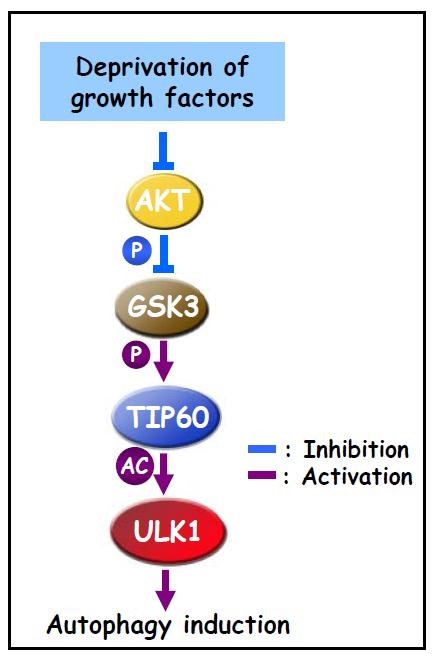
近年来,林圣彩教授以细胞代谢稳态调控为研究核心,针对细胞对营养物质与能量的感知机制以及代谢紊乱相关疾病的发生发展的分子机制进行研究,并取得了一系列原创性成果。揭示了生长因子缺乏诱导细胞启动自噬的分子机制(Science, 2012),及其对糖代谢流的调控(Molecular Cell, 2016);发现和鉴定了细胞感应葡萄糖缺乏的溶酶体途径和“葡萄糖感受器”,及其激活AMPK的方式,并打破了传统的“AMPK的激活仅依赖于AMP浓度的变化”的认知(Cell Metabolism, 2013, 2014; Nature, 2017)。此外,还揭示了自己团队发现的AIDA基因在哺乳动物中作为脂肪吸收、合成和储存相关的“浪费基因”的生理功能、及其蛋白质通过内质网降解(ERAD)途径控制膳食脂肪吸收过程的重要功能(Cell Metabolism, 2018),和甘油磷酸酶lipin1在甘油三酯合成中的关键作用(Nature Communications,2018)。他与DG Hardie作为共同通讯作者在发表了有关AMPK激活机制与功能综述文章(Cell Metabolism,2018),和有关碳水化合物在抗氧化方面的作用的评论文章(Cell Metabolism,2018)。林圣彩教授早前的成就还体现在解释了两种侏儒症的发生机制 (Nature, 1991; Nature, 1993),以及AXIN作为架构蛋白质介导抑癌因子p53等的激活机制(EMBO, 2005; Nature Cell Biology, 2009)。 Our research lies in the broad area of the molecular mechanisms that underlie metabolic homeostasis and its relationship to cell growth control. We have discovered a signaling pathway comprising the protein kinase GSK3, acetyltransferase TIP60, and protein kinase ULK1, which activates autophagy in cells deprived of serum, elucidating the molecular mechanism linking nutritional starvation to autophagy. We have also revealed that the ULK kinases phosphorylate multiple glycolytic enzymes and regulate glucose metabolic fluxes dependently of autophagy. The main focus of our research has also been on the mechanisms for the regulation of the AMP-activated protein kinase. We have shown that the lowenergy signal AMP can autonomously initiate assembly of an activating complex for the energy sensor kinase AMPK, in that the scaffold protein AXIN tethers LKB1 to AMPK. Moreover, we have found that the AXIN/LKB1/AMPK complex and mTORC1 inversely utilize the common v-ATPaseRagulator complex for activation on the surface of lysosome, thereby uncovering a switch between anabolism and catabolism. We are exploring further how AMPK and its activating kinase LKB1 are regulated and the biological functions of these kinases.
代表性论文(Selected Publications)
1. Lin SY#, Li TY#, Liu Q, Zhang C, Li X, Chen Y, Zhang SM, Lian G, Liu Q, Ruan K, Wang Z, Zhang CS, Chien KY, Wu J, Li Q, Han J, Lin SC*. GSK3-TIP60-ULK1 signaling pathway links growth factor deprivation to autophagy. Science, 336(6080)(2012), 477-481.
2. Zhang YL#, Guo H#, Zhang CS#, Lin SY, Yin Z, Peng Y, Luo H, Shi Y, Lian G, Zhang C, Li M, Ye Z, Ye J, Han J, Li P, Wu JW, Lin SC*. AMP as a low-energy charge signal autonomously initiates assembly of AXIN-AMPK-LKB1 complex for AMPK activation. Cell Metabolism, 18(4)(2013), 546-555.
3. Zhang CS#, Jiang B#, Li M#, Zhu M, Peng Y, Zhang YL, Wu YQ, Li TY, Liang Y, Lu Z, Lian G, Liu Q, Guo H, Yin Z, Ye Z, Han J, Wu JW, Yin H, Lin SY, Lin SC*. The lysosomal v-ATPase-Ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. Cell Metabolism., 20(3)(2014), 526-540.
4. Li TY, Sun Y, Liang Y, Liu Q, Shi Y, Zhang CS, Zhang C, Song L, Zhang P, Zhang X, Li X, Chen T, Huang HY, He X, Wang Y, Wu YQ, Chen S, Jiang M, Chen C, Xie C, Yang JY, Lin Y, Zhao S, Ye Z, Lin SY, Chiu DT, Lin SC*. ULK1/2 Constitute a Bifurcate Node Controlling Glucose Metabolic Fluxes in Addition to Autophagy. Moleculer Cell, 62(3)(2016), 359-370.
5. Zhang CS#, Li M#, Ma T#, Zong Y, Cui J, Feng JW, Wu YQ, Lin SY, Lin SC*. Metformin Activates AMPK through the Lysosomal Pathway. Cell Metabolism, 24(4)(2016), 521-522.
6. Zhang CS#, Hawley SA#, Zong Y#, Li M#, Wang Z, Gray A, Ma T, Cui J, Feng JW, Zhu M, Wu YQ, Li TY, Ye Z, Lin SY, Yin H, Piao HL, Hardie DG*, Lin SC*. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature, 548(7665)(2017), 112-116.
7. Luo H#, Jiang M#, Lian G, Liu Q, Shi M, Li TY, Song L, Ye J, He Y, Yao L, Zhang C, Lin ZZ, Zhang CS, Zhao TJ, Jia WP, Li P, Lin SY*, Lin SC*. AIDA Selectively Mediates Downregulation of Fat Synthesis Enzymes by ERAD to Retard Intestinal Fat Absorption and Prevent Obesity. Cell Metabolism, 27(4)(2018), 843-853.
8. Li M#, Zhang CS#, Zong Y#, Feng JW, Ma T, Hu M, Lin Z, Li X, Xie C, Wu Y, Jiang D, Li Y, Zhang C, Tian X, Wang W, Yang Y, Chen J, Cui J, Wu YQ, Chen X, Liu QF, Wu J, Lin SY, Ye Z, Liu Y, Piao HL, Yu L, Zhou Z, Xie XS, Hardie DG, Lin SC*. Transient Receptor Potential V Channels Are Essential for Glucose Sensing by Aldolase and AMPK. Cell Metabolism, 30(3)(2019), 508-524.
9. Zong Y#, Zhang CS#, Li M#, Wang W#, Wang Z, Hawley SA, Ma T, Feng JW, Tian X, Qi Q, Wu YQ, Zhang C, Ye Z, Lin SY, Piao HL, Hardie DG, Lin SC*. Hierarchical activation of compartmentalized pools of AMPK depends on severity of nutrient or energy stress. Cell Research, 29(6)(2019), 460-473.
10. Shi M#, Huang XY#, Ren XY, Wei XY, Ma Y, Lin ZZ, Liu DT, Song L, Zhao TJ, Li G, Yao L, Zhu M, Zhang C, Xie C, Wu Y, Wu HM, Fan LP, Ou J, Zhan YH, Lin SY*, Lin SC*. AIDA directly connects sympathetic innervation to adaptive thermogenesis by UCP1. Nature Cell Biololgy, 23(3)(2021), 268-277.