韩爱东HAN Aidong, Ph.D.

教授,博士生导师
E-mail: ahan@xmu.edu.cn
1985年9月-1989年7月, 南京大学/生物系, 学士学位
1992年9月-1995年7月, 中山大学/遗传工程中心, 硕士学位
1995年5月-1999年8月, 中国科学学院微生物研究所,博士学位
1999年9月-2006年5月,美国科罗拉多大学博德分校/生化系, 访问学者
2006年6月-2008年1月,美国南加州大学/分子与计算生物系, 访问学者
1999, Ph.D., Institute of Microbiology, Chinese Academy of Sciences;
1999-2006, Postdoctoral follow, University of Colorado at Boulder;
2006-2008, Senior research associate, University of Southern California.
研究领域(Research Area)
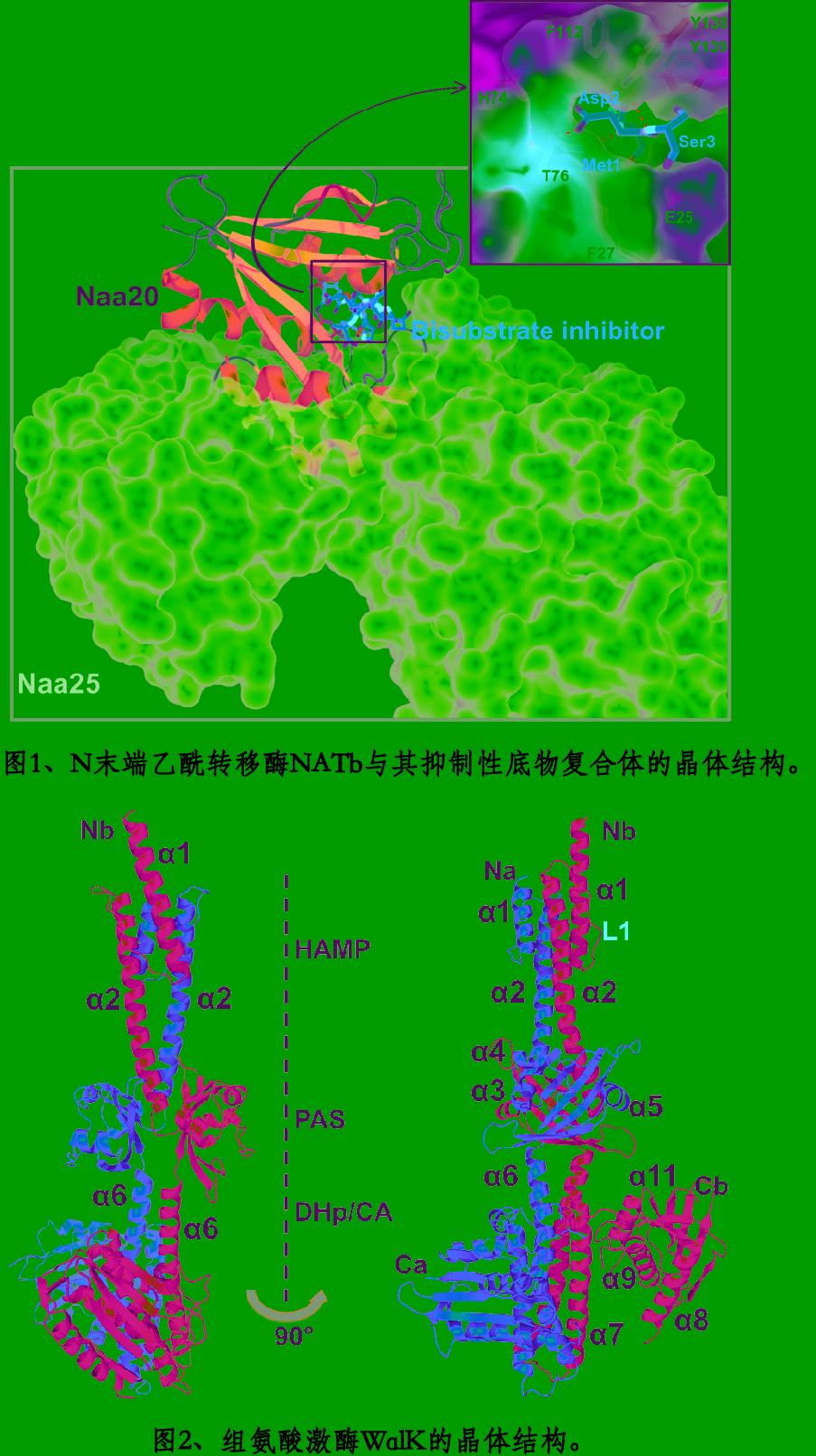
课题组以结构生物学为主要的研究手段,揭示细胞的信号转导、物质运输、蛋白质翻译后修饰、与微生物相互作用等生理活动的分子机制。 在真核细胞中,蛋白质的乙酰化修饰通常发生在N末端和赖氨酸侧链上,是调节蛋白质功能,稳定性和细胞内定位的重要手段。N末端乙酰转移酶(NAT)负责蛋白质N末端乙酰化修饰(图1)。组蛋白乙酰转移酶(HAT)和组蛋白去乙酰化酶(HDAC)负责赖氨酸侧链的乙酰化修饰。蛋白质的磷酸化修饰具有更广泛的表型和功能。我们正在多方面地研究tau蛋白的修饰、转运以及在神经退行性疾病中的作用机制等,并开展相关药物的设计和研发。
在原核细胞中,组氨酸激酶(Sensor histidine Kinase, SK)负责刺激依赖的组氨酸磷酸化,并将信号传导至相关的调节蛋白(Response Regulator, RR),以调控各种各样的生物学过程。这一调控系统被称作双组分系统(Two-Component System, TCS)。基因表达的转录调控是TCS的一个主要功能 。一些常见的革兰氏阳性细菌引起脑膜炎、皮肤炎、鼻竇炎、肺炎等感染性疾病,又容易产生抗生素的耐药性。WalRK, 也叫VicRK或YycFG是这些细菌中关键的TCS。课题组解析了完整的胞内WalK的晶体结构(图2),正试图解析带有细胞外感应结构域的全长WalRK复合体结构,以探索HAMP和PAS等结构域介导的信号转导和激酶活性调控机制,并积极研发新型抗生素。
My lab is interested in understanding molecular mechanisms of several key biological processes, including signal transduction through membrane, intracellular trafficking, protein post-translational modification (PTM), and microbe-host interaction. Protein acetylation, one of important PTMs, regulates protein function, stability and cellular localizations in eukaryotic cells. Proteins can be acetylated at the amine group of peptidic N-terminus and lysine side chain. A group of proteins called N-terminal acetyltransferases (NAT) acetylate protein N-terminus (Fig. 1). The lysine acetylation as one of important means for epigenetic regulations in transcription is carried out reversibly by histone acetyltransferases (HAT) and histone deacetylases (HDAC), which often form high-order regulatory complexes. It is well known that protein phosphorylation by a variety of protein kinases play key roles in even broader biological processes. We are focusing our attention on tau phosphorylation, hoping to further our current understanding on its spreading and roles in neurodegenerative diseases. In prokaryotic cells, two-component system (TCS), including histidine kinase (SK) and response regulator (RR), is responsible for a diverse of biological processes, one of which is stimuli induced transcription. Our lab uses WalRK, also called VicRK or YycFG, an essential TCS in several Gram-positive bacteria as a model system to understand how intra/extracellular signals regulate the gene transcription for bacterial survival under stress environments. With crystal or CroEM structures (Fig. 2), we hope to develop leading compounds for new antibiotics that may overcome the rapidly progressing antibiotic resistance in these pathogenic bacteria.
代表性论文(Selected Publications)
8. Jayathilaka, N, Han, A , Gaffney, KJ, Dey, R, Jarusiewicz, JA, Noridomi, K, Philips, MA, Lei, X, He, J, Ye, J, Gao, T, Petasis, NA and Chen, L (2012). Inhibition of the function of class IIa HDACs by blocking their interaction with Mef2. Nucleic Acids Research 40(12): 5378-5388.
参加学术团体的情况(Professional service)
中国生物化学和分子生物学会会员
中国生物物理学会会员
美国心脏和中风学会会员
国际期刊NAR,JMB,JBC等的审稿人